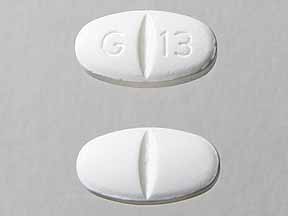
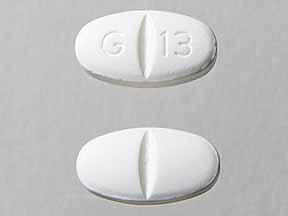
Pill with imprint G 13 is White, Oval and has been identified as Gabapentin 800 mg. It is supplied by Glenmark Generics Inc.
Gabapentin is used in the treatment of Back Pain; Postherpetic Neuralgia; Epilepsy; Chronic Pain; Seizures and belongs to the drug class gamma-aminobutyric acid analogs. Risk cannot be ruled out during pregnancy. Gabapentin 800 mg is not a controlled substance under the Controlled Substances Act (CSA).
Gabapentin
- Imprint
- G 13
- Strength
- 800 mg
- Color
- White
- Size
- 19.00 mm
- Shape
- Oval
- Availability
- Prescription only
- Drug Class
- Gamma-aminobutyric acid analogs
- Pregnancy Category
- C – Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Glenmark Generics Inc.
- Inactive Ingredients
- corn starch, copovidone, poloxamer 407, magnesium stearate, titanium dioxide, magnesium silicate, polysorbate 80, water
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 68462-0127 | Glenmark Pharmaceuticals Inc. |
| 54868-5195 (Discontinued) | Physicians Total Care Inc. (repackager) |
What is Gabapentin and How to Use it ?
Gabapentin is a medication primarily used to treat seizures (epilepsy) and nerve pain (neuropathic pain). It belongs to a class of medications known as anticonvulsants or antiepileptic drugs. Gabapentin works by affecting certain chemicals and neurotransmitters in the brain involved in seizures and nerve pain.
Here’s how gabapentin is typically used:
- Seizures (Epilepsy): Gabapentin is commonly prescribed as an adjunctive treatment for partial seizures in adults and children aged 3 years and older. It is often used in combination with other antiepileptic medications to help control seizures.
- Nerve Pain (Neuropathic Pain): Gabapentin is also frequently used to treat various types of nerve pain, including diabetic neuropathy, postherpetic neuralgia (nerve pain following shingles), and peripheral neuropathy. It is effective in relieving shooting or burning sensations, tingling, or numbness associated with nerve damage.
Gabapentin is usually taken orally in the form of capsules, tablets, or an oral solution. Here are some general guidelines for using gabapentin:
- Dosage: The dosage of gabapentin varies depending on the individual’s medical condition, age, and response to treatment. It’s essential to follow your doctor’s instructions and the dosage prescribed on the medication label carefully.
- Administration: Gabapentin is typically taken by mouth with or without food. Swallow the capsule or tablet whole with a full glass of water. Do not crush, chew, or break the tablet or capsule unless directed to do so by your doctor.
- Timing: Take gabapentin exactly as prescribed by your doctor. It’s important to take it regularly and at the same times each day to maintain a consistent level of the medication in your body.
- Gradual Dosing: If you are starting gabapentin treatment or increasing the dosage, your doctor may recommend starting with a low dose and gradually increasing it over time to minimize side effects.
- Duration of Treatment: Continue taking gabapentin for the full duration of treatment prescribed by your doctor, even if you start to feel better. Stopping gabapentin suddenly can increase the risk of seizures or other withdrawal symptoms.
- Monitor Side Effects: Pay attention to any side effects you may experience while taking gabapentin and report them to your doctor. Common side effects may include dizziness, drowsiness, fatigue, and weight gain.
- Storage: Store gabapentin at room temperature away from moisture and heat. Keep it out of reach of children and pets.
It’s important to note that gabapentin can interact with other medications, so it’s essential to inform your doctor about all the medications you are taking, including prescription drugs, over-the-counter medications, and supplements. Additionally, never adjust your gabapentin dosage or stop taking it without consulting your doctor first, as sudden changes can lead to serious complications.
Glenmark Generics Inc.
Glenmark Generics Inc. is a subsidiary of Glenmark Pharmaceuticals Ltd., an Indian multinational pharmaceutical company headquartered in Mumbai, India. Glenmark Generics focuses on developing, manufacturing, and distributing generic pharmaceutical products for the U.S. and other international markets. Here is a detailed overview of Glenmark Generics Inc.:
Company Overview
- Parent Company: Glenmark Pharmaceuticals Ltd.
- Founded: 1977 by Gracias Saldanha.
- Headquarters: Mumbai, Maharashtra, India.
- Global Presence: Glenmark Pharmaceuticals operates in over 50 countries with a strong presence in North America, Europe, and Asia.
- Glenmark Generics Inc.:
- Focus: Specializes in the development, manufacture, and distribution of generic pharmaceutical products.
- Market: Primarily targets the U.S. market, one of the largest and most regulated pharmaceutical markets globally.
- Product Portfolio:
- Generics: Glenmark Generics offers a wide range of generic medications across various therapeutic categories, including dermatology, respiratory, oncology, cardiovascular, and central nervous system (CNS) disorders.
- Specialty Products: The company also works on developing specialty and complex generics, including injectables and inhalation products.
- Manufacturing and R&D:
- Facilities: Glenmark has multiple manufacturing facilities in India and other countries that comply with international regulatory standards such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and others.
- Research and Development: Significant investment in R&D to develop new generic formulations and improve existing ones. Glenmark has several R&D centers globally, including a notable facility in Mahape, Navi Mumbai.
- Regulatory Compliance:
- Quality Standards: Glenmark Generics ensures adherence to stringent quality control and regulatory standards set by global regulatory bodies, including the FDA.
- Inspections and Approvals: The company regularly undergoes inspections and audits to maintain compliance with Good Manufacturing Practices (GMP).
- Market Strategy:
- Product Launches: Glenmark Generics is known for timely launches of first-to-file generic products, which can be highly profitable due to market exclusivity periods.
- Competitive Pricing: Focus on providing cost-effective alternatives to brand-name medications, making healthcare more affordable.
- Acquisitions and Partnerships:
- Glenmark Pharmaceuticals has expanded its generics business through strategic acquisitions and partnerships, enhancing its product portfolio and market reach.
- Corporate Responsibility:
- Sustainability: Glenmark is committed to sustainable practices in its operations, focusing on environmental protection, efficient use of resources, and reducing its carbon footprint.
- Community Engagement: The company engages in various corporate social responsibility (CSR) activities, including healthcare initiatives, education, and community development projects.
Key Achievements and Initiatives
- Innovation in Generics: Glenmark Generics has introduced several first-to-market generics, providing affordable alternatives to expensive brand-name drugs.
- Global Expansion: The company has significantly expanded its footprint in the U.S. and other international markets, establishing itself as a reputable player in the generic pharmaceuticals industry.
- R&D Excellence: With a strong focus on research and development, Glenmark has developed numerous complex generics and innovative drug delivery systems.
Conclusion
Glenmark Generics Inc., a subsidiary of Glenmark Pharmaceuticals Ltd., plays a crucial role in the global generics market, particularly in the U.S. With a broad product portfolio, rigorous regulatory compliance, and a strong commitment to quality and innovation, Glenmark Generics continues to be a significant contributor to making healthcare more accessible and affordable worldwide. The company’s ongoing efforts in research and development, along with strategic market strategies, position it well for continued growth and success in the pharmaceutical industry.