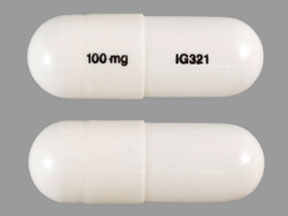
Pill with imprint 100 mg IG321 is White, Capsule/Oblong and has been identified as Gabapentin 100 mg. It is supplied by InvaGen Pharmaceuticals, Inc.
Gabapentin is used in the treatment of Back Pain; Postherpetic Neuralgia; Epilepsy; Chronic Pain; Seizures and belongs to the drug class gamma-aminobutyric acid analogs. Risk cannot be ruled out during pregnancy. Gabapentin 100 mg is not a controlled substance under the Controlled Substances Act (CSA).
Images for 100 mg IG321
Gabapentin 100 mg IG321
- Imprint
- 100 mg IG321
- Strength
- 100 mg
- Color
- White
- Size
- 16.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- Gamma-aminobutyric acid analogs
- Pregnancy Category
- C – Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- InvaGen Pharmaceuticals, Inc.
- National Drug Code (NDC)
- 31722-0221
- Inactive Ingredients
- mannitol, corn starch, magnesium silicate, FD&C Red No. 40, D&C Yellow No. 10, titanium dioxide
Note: Inactive ingredients may vary.
What is Gabapentin ?
Gabapentin is a medication primarily used to treat epilepsy and neuropathic pain. It is also commonly prescribed for other conditions such as restless legs syndrome (RLS) and certain anxiety disorders. Here is a detailed overview of gabapentin:
Mechanism of Action
Gabapentin’s exact mechanism of action is not completely understood, but it is known to interact with the α2δ subunit of voltage-gated calcium channels in the central nervous system. This interaction reduces the release of excitatory neurotransmitters, which helps to stabilize neuronal activity and alleviate symptoms associated with neuropathic pain and seizures.
Therapeutic Uses
- Epilepsy: Gabapentin is used as an adjunctive therapy in the treatment of partial seizures with or without secondary generalization in adults and children over the age of 3.
- Neuropathic Pain:Effective in managing pain from conditions such as diabetic neuropathy, postherpetic neuralgia (pain following shingles), and other forms of nerve pain.
- Restless Legs Syndrome (RLS):Gabapentin can help reduce the symptoms of RLS, improving sleep quality and reducing leg discomfort.
- Anxiety Disorders:Sometimes used off-label for the treatment of anxiety disorders, including generalized anxiety disorder (GAD) and social anxiety disorder (SAD).
- Fibromyalgia:Can be used to manage the chronic pain associated with fibromyalgia.
- Seizure Management: Gabapentin may be used alongside other medications to reduce seizure frequency in adults and children aged three and older who experience partial-onset seizures.
- Postherpetic Neuralgia: In adults, gabapentin is effective for managing postherpetic neuralgia, which is persistent nerve pain following a Shingles infection.
- Horizant (Gabapentin Enacarbil): This long-acting prodrug of gabapentin is taken once daily, usually around 5 PM, to relieve restless legs syndrome (RLS) or nerve pain associated with postherpetic neuralgia.
- Generic Availability: Gabapentin is available in generic forms, though not all generics are interchangeable with some branded versions.
- Off-Label Uses: Gabapentin may also be prescribed off-label for conditions such as fibromyalgia, persistent hiccups, migraine prevention, and hot flashes. Different brands and formulations of gabapentin exist, each with specific uses and dosing instructions.
- Neurontin: Used to treat pain from shingles (postherpetic neuralgia) and, when combined with other seizure medications, treats partial-onset seizures in adults and children over three years old.
- Gralise: Specifically designed for treating postherpetic neuralgia and should not be used for other conditions.
- Horizant: An extended-release tablet intended for restless legs syndrome and postherpetic neuralgia.
- Generic Gabapentin Capsules: Used for treating postherpetic neuralgia and as an additional therapy for partial-onset seizures in adults and children over three years old.
Pharmacokinetics
- Absorption:
- Gabapentin is absorbed from the gastrointestinal tract, with peak plasma concentrations occurring within 2 to 3 hours after oral administration. Its bioavailability decreases as the dose increases.
- Distribution:
- Widely distributed throughout the body but does not significantly bind to plasma proteins.
- Metabolism:
- Unlike many other medications, gabapentin is not significantly metabolized in the liver.
- Elimination:
- Excreted unchanged by the kidneys. The half-life of gabapentin is about 5 to 7 hours in individuals with normal renal function.
Side Effects
Common side effects of gabapentin include:
- Dizziness
- Drowsiness
- Peripheral edema (swelling of the extremities)
- Fatigue
- Ataxia (lack of coordination)
- Nystagmus (involuntary eye movement)
Less common but more serious side effects can include:
- Mood changes such as depression or anxiety
- Suicidal thoughts or behaviors
- Severe allergic reactions (rare)
Dosage
Gabapentin is available in various forms, including capsules, tablets, and an oral solution. Dosage depends on the condition being treated and individual patient factors, but it typically starts at a low dose and is gradually increased. Common dosing regimens include:
- Epilepsy: Starting at 300 mg once daily and increasing based on response.
- Neuropathic Pain: Starting at 100-300 mg once daily and titrating up as needed.
- RLS: Typically 600 mg once daily in the evening.
Drug Interactions
Gabapentin has relatively few drug interactions compared to many other medications, but it can interact with:
- Antacids (reduce gabapentin’s absorption)
- CNS depressants (increased risk of sedation and respiratory depression)
InvaGen Pharmaceuticals, Inc
InvaGen Pharmaceuticals, Inc. is a generic pharmaceutical company that develops, manufactures, and markets a wide range of affordable and high-quality generic medications. It is a subsidiary of the global pharmaceutical company, Cipla Ltd., based in India. Here is an overview of InvaGen Pharmaceuticals, Inc.:
Company Overview
- Parent Company:
- Cipla Ltd.: InvaGen Pharmaceuticals is a subsidiary of Cipla Ltd., a leading global pharmaceutical company headquartered in Mumbai, India. Cipla is known for its commitment to providing access to affordable and high-quality medicines.
- Headquarters:
- Location: Hauppauge, New York, USA.
Operations and Facilities
- Manufacturing:
- InvaGen Pharmaceuticals operates manufacturing facilities that are designed to comply with stringent regulatory standards, including those of the U.S. Food and Drug Administration (FDA).
- The company’s facilities are equipped with advanced technology and have significant production capacities to meet both domestic and international demand.
- Research and Development:
- InvaGen focuses on research and development to bring new generic formulations to market and improve existing products.
- The company’s R&D efforts are aimed at ensuring the highest standards of product quality, safety, and efficacy.
Product Portfolio
- Generics:
- InvaGen Pharmaceuticals offers a diverse range of generic medications across various therapeutic categories, including cardiovascular, central nervous system, anti-infective, gastrointestinal, and pain management drugs.
- The company provides cost-effective generic alternatives to brand-name medications, making them accessible to a broader patient population.
Key Areas of Operation
- Regulatory Compliance:
- Quality Assurance: InvaGen adheres to rigorous quality control measures and regulatory requirements set by the FDA and other global regulatory bodies.
- GMP Compliance: The company ensures that its manufacturing processes comply with Good Manufacturing Practices (GMP) to guarantee the safety, efficacy, and quality of its products.
- Market Strategy:
- Product Launches: InvaGen focuses on the timely launch of generic medications to capture market share and provide affordable options to patients.
- Competitive Pricing: The company’s competitive pricing strategy ensures that its products are accessible to a broad patient population.
- Partnerships and Collaborations:
- Expansion: InvaGen pursues strategic partnerships and collaborations to enhance its product offerings and expand its market reach.
- Distribution Networks: The company has established robust distribution networks to ensure the widespread availability of its products.
Strategic Growth
- Acquisitions and Investments:
- Cipla Ltd.’s acquisition of InvaGen Pharmaceuticals has strengthened Cipla’s presence in the U.S. market and expanded its global footprint.
- Ongoing investments in manufacturing and R&D facilities ensure that InvaGen remains at the forefront of the generic pharmaceutical industry.
- Innovation:
- InvaGen continues to invest in the development of complex generics and novel drug delivery systems to address unmet medical needs and provide innovative solutions for patients.
Corporate Responsibility
- Sustainability:
- The company is committed to sustainable manufacturing practices, focusing on minimizing its environmental impact through efficient resource use and waste management.
- Community Engagement:
- InvaGen participates in various corporate social responsibility (CSR) initiatives aimed at improving healthcare access, supporting education, and contributing to community development.